What is the problem
Most drugs for cancer are approved based on randomized trials. Recent FDA programs have allowed promising drugs to get to market faster, but with sometimes questionable effectiveness. We use cutting-edge techniques in observational data analysis to study "real-world" prescribing patterns and effectiveness of oncology therapeutics
What are we doing
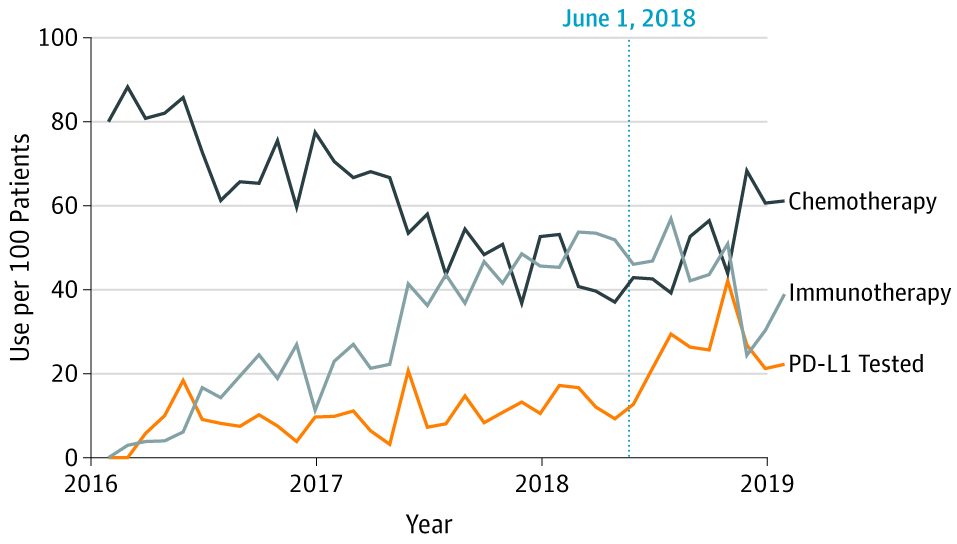
Through a collaboration with Flatiron Health, we used real-world data to study the impact of unprecedented FDA label restrictions on prescribing of immunotherapies. In another Flatiron collaboration, we are studying the effectiveness of novel cancer therapeutics in populations traditionally excluded from clinical trials. Finally, we have studied the use of high-cost oncology therapeutics in non-evidence-based indications and at the end of life.
Impact
Our evidence generated has informed critical analysis of the FDA's accelerated approval program and have contributed to the debate for expanding trial eligiblity criteria for older and unfit populations.

