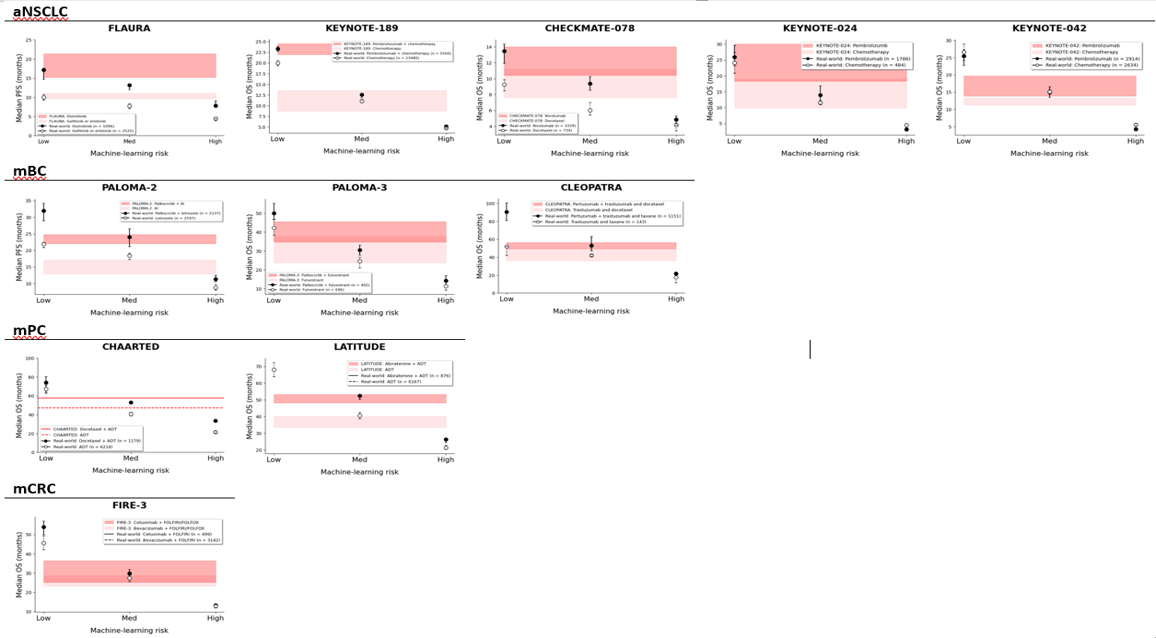
Clinical trials are the gold standard for approving oncology drugs. But only 10% of the US population will ever participate in a clinical trial, and trials are oftentimes expensive and take time to read out. How can we use cutting-edge techniques in causal inference and machine learning to simulate in-silico clinical trials for novel therapeutics and in underrepresented patient populations? In one series of projects i, we are using machine learning algorithms to simulate in-silico trials of novel agents in breast, lung, and other cancers, to identify subpopulations that may not respond as favorably as cited in clinical trials. We have also simulated race-based differences in effectiveness of two common prostate cancer drugs. We have ongoing mixed-methods studies to identify best practices for digital twins and in silico trials. There is a large debate about how observational data should be used for oncology drug approvals. We aim to produce high-quality evidence that establishes methodologic frameworks for in-silico trials. While we do not argue for replacing clinical trials, we believe that in-silico trials are a crucial adjunct to bring the most effective agents to phase III testing and to market.



