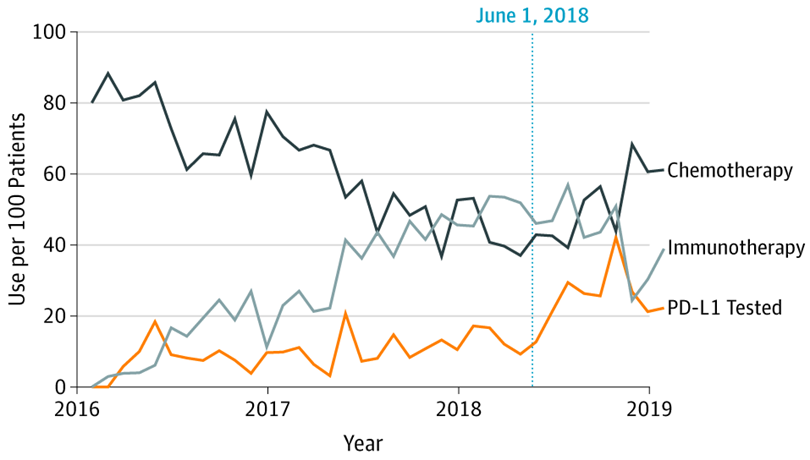
Through unique access to both claims-based and electronic health record data sources, we have established a robust portfolio in comparative effectiveness and policy analyses. In one of the first analyses using a real-world data source to study changes in drug utilization, we published a study in JAMA that has been widely cited in reforms proposed to the FDA accelerated approval programs. We have also published a number of analyses comparing novel therapeutics to inform real-world clinical decision-making in genitourinary and thoracic malignancies.
Research Categories

